Density of Nacl Solution
Sodium chloride is also known as salt. How many grams of NaCl are needed to make 159 L of a 0531 M solution 204 ML of 43 7M HCl A.

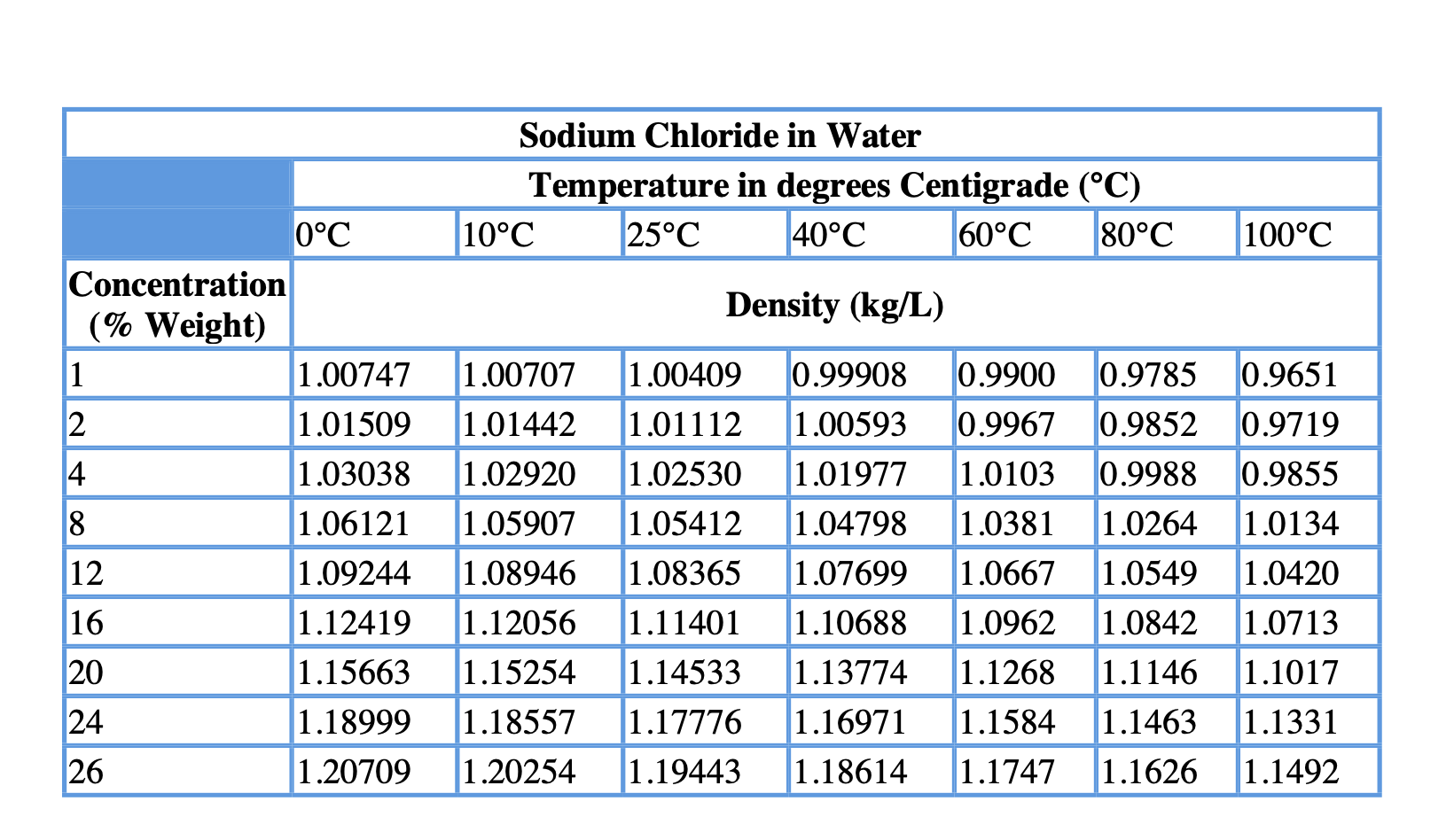
Variation Of Nacl Aqueous Solutions Density With Temperature And Nacl Download Table
Determine the mole fraction of NaCl in a solution in which 010 moles of the salt is dissolved in 100 grams of water.

. Small amount is bolded to stress the fact that if you add too much strong acid or base to your buffer its pH will change. NaCl and KCl were used as molten salts and the cathode material synthesized with NaCl exhibited more isotropic morphologies than the material synthesized with KCl. The density of the resulting solution is considered to be equal to that of water statement holding especially for dilute solutions so the density information is not required.
Expressed as ww wt and for density in many cases. K sp Ag Br- 50 x 10-13. We can generate a second equation however by noting that one Ag ion is released for every Br-ion.
Based on ASTM G109 a 3 by weight salt solution was injected into the upper cap while 99 pure sodium chloride NaCl was used to minimize the possibility of interference from other ions. Extract the data from the question mass of solute volume of. In the membrane process a solution of approximately 33.
Interestingly the dissolved salt does not increase the volume of the water by the volume of the added salt and this is due to the. When salt sodium chloride or NaCl dissolves in water there is a significant increase in mass of the solution due to the relatively higher molecular mass of the dissolved ions Na 22 gmol and Cl 355gmol when compared to water or H 2 O 20 gmol. A buffer is a solution that resists changes in its pH when small amounts of strong acid or base is added to it.
Molecular weight of NaCl 5844. Q solution m c T where m is the total mass of the resultant solution and c is the specific heat capacity of the resultant solution. I density of each dilute aqueous solution is the same as water 1 g mL-1 at 25C so the mass of solution in grams volume of solution in mL ii heat capacity of each solution is the same as for water C g 418 JC-1 g-1.
In a solution there is 1110 mL 110605 g solvent and 524 mL 60508 g solute present in a solution. Total solution volume is known same equation as case 1. Mass of 1 litre of solution 125 gmsml 1000 ml 1 250 gms V volume of water added to make the solution or volume of solvent Mass of solute Mass of solvent Mass of solution 17532 gms mass of solvent 1 250 gm mass of solvent 1250-17532 10748 So 1 07468 gms of water is mixed with 3 moles of NaCl to make the 3 M solution.
Pressure of Gas - Measured in Pascal - The pressure of Gas is the force that the gas exerts on the walls of its container. Calculating the molar enthalpy of neutralisation using the data from the experiment. M B C V A 100.
The density of a 3M solution of NaCl is 125gml. A 15L solution is composed of 025g NaCl dissolved in water. This means buffer solutions.
It is also found as rock salt. Density of sodium chloride NaClaq Table 1. The production of caustic soda NaOH also results in the co-products of chlorine and hydrogen.
H 101 gmol. One equation cant be solved for two unknowns the Ag and Br-ion concentrations. Density of Gas - Measured in Kilogram per Meter³ - The Density of Gas is defined as mass per unit volume of a gas under specific conditions of temperature and pressure.
The electrons in the. Estimated values of absolute density gcm 3 of aqueous sodium chloride solutions NaCl H 2 O as function of electrolyte molality m and temperature T at pressure p 1 bar. In its aqueous form it is called a saline solution.
It is a crystalline solid white. Expressed as vv when mixture or solute is liquid. Calculate the molality of the NaCl.
3 Molar solution means there are 3 moles of NaCl salt in 1 Liter. The moles of NaCl is provided but you still need the number of moles of water H 2 O. What is it and how does it work to resist changes in its pH.
Find the mass percent volume percent and massvolume percent of the solute. It occurs in oceans and sea waters. Density v o l u m e m a s s Mass of 1 litre of solution 125 gmsml 1000 ml 1 250 gms.
Hence there are 3 5 8. Since the solutions are mostly water the solutions are assumed to have a density of 10 gmL and a specific heat of 418 JgC. The molecular weight of NaCl is 5844gmol.
Weight versus volume percent concentration g number of solute in 100m of solution. When the chloride solution penetrates the concrete and reaches the upper rebar oxidation reactions occur in the upper rebar anode. Atomic mass Na 2299 atomic mass Cl 3545 moles of NaCl 3165 g x 1 mol2299 3545 moles of NaCl 3165 g x 1 mol5844 g moles of NaCl 0542 mol kg water density x volume.
We then write the solubility product expression for this reaction. 4 4 gms in 1 Litre of water. Gram equivalent number of solute in 1L solution.
A Given Volume 159 Liter Molarity 0531 Mole Liter mass of NaCl. Sodium bisulfate also known as sodium hydrogen sulfate is the sodium salt of the bisulfate anion with the molecular formula NaHSO 4Sodium bisulfate is an acid salt formed by partial neutralization of sulfuric acid by an equivalent of sodium base typically in the form of either sodium hydroxide lye or sodium chloride table salt. Molar Mass - Measured in Kilogram Per Mole - Molar Mass is the mass of a given.
About 1 to 5 of seawater is made of NaCl. Molality m of NaCl moles of NaClkg water From the periodic table find the atomic masses of the elements. Values in the table are consistent with the density of pure water calculated with the IAPWS-95 equation of state.
Volume percent concentration m number of solute in 100m solution. It is a dry granular product that can be. The heat gained by the resultant solution can be calculated using.
Prepared from sodium chloride NaCl is electrolyzed in either a membrane cell or a diaphragm cell. Make 2 g100mL of NaCl solution with 1 L water Water properties. Start by calculating the number of moles in one gram of water using periodic table data for hydrogen and oxygen.
The most consistent trial is Trial 4. Calculate the molality of the solution. Synthesized LiNi 08 Co 01 Mn 01 O 2 cathode material using NaCl and KCl using the molten-salt method and the particles using NaCl exhibited 101 and 003 facets with almost perfect.
Either wv wv 100 or mv mv 100 weightvolume mass solute volume of solution 100. Sodium chloride is an ionic compound with the chemical formula NaCl. Identify the solute and solvent by name or chemical formula solute sodium chloride NaCl solvent is water H 2 O because this is an aqueous solution.
Because there is no other source of either ion in this solution the concentrations of these ions. With the solution shown in the picture below find the mole percent of substance C. The following table shows data from the titration of four 100 mL samples of a solution containing A.
Solved What Is The Density Of 22 Nacl Solution At 50 Chegg Com

Variation Of Nacl Aqueous Solutions Density With Temperature And Nacl Download Table

Variation Of Nacl Aqueous Solutions Density With Temperature And Nacl Download Table
0 Response to "Density of Nacl Solution"
Post a Comment